Characteristics of Sulfuric Acid
Sulfuric acid is a potent dibasic and diprotic acid that ionises in the aqueous solution in two stages. Fast Delivery Next Day Shipping on Most Orders and Access to 247 Knowledgeable Support.

Physical And Chemical Properties Of Sulfuric Acid Download Table
Chemical Properties of Sulphuric Acid.

. Pure sulfuric acid is a colourless dense heavy and syrupy oily liquid. H 2 SO 4 is a strong acid that completely dissociates into ions in its aqueous solution as H 2 SO 4 H 2 SO 4-2. Sulfuric Acid I 4 Physical Properties Sulfuric acid H 2 SO 4 Molecular Weight 9808 is a heavy oily strong liquid mineral acid with a pH less than 1 and an acrid odor.
1 Action of Heat- On heating strongly it undergoes dissociation at 440C. Sulfuric acid is soluble in water but generates heat. Ad Find sulfuric Acid and Over 15 Million Products at Grainger Today.
184 gmL at 298 K. Sulfuric acids varied properties of acidity reactivity and corrosiveness its sulfur content and its affinity for water play essential roles in the manufacture of products such as fertilizers paints. Sulfuric acid is aliquidoily colorless and peculiar smell depending on its concentrationIt has a melting point of 10 C and a boiling point of 337 C.
The sulphuric acid obtained in this process is over 96 pure. With increasing attention on sulfuric acid emission investigations on the removal characteristics of sulfuric acid aerosols by the limestone gypsum wet flue gas desulfurization WFGD system. For instance the blue copper salt copperII.
It is a highly corrosive substance with strong reactivity. High boiling point and. Contact Us for a Quote Now.
Chemical Properties of Sulfuric Acid. Chemically sulfuric acid is an important reactive compound. Sulphuric Acid is a.
Its density is 184g cm3It is miscible soluble with water but generates heat given its en See more. This chemical is highly corrosive reactive. The Chemical Properties of Sulfuric acid.
Sulphuric acid often known as sulfuric acid or H2SO4 is an oily odourless liquid. The boiling point of the Sulphuric acid is 611. Physical Properties of Sulfuric Acid.
Learn Sulfuric Acid formula structure uses Embibe. ChemDirect Powers The Best Chemical Buying Selling Experience Online. It is completely soluble.
Sulphuric acid is a thick oily fluid and is colorless. Sulfuric Acid I 4 Physical Properties Sulfuric acid H 2 SO 4 Molecular Weight 9808 is a heavy oily strong liquid mineral acid with a pH less than 1 and an acrid odor. Because the molar concentration of 1 sulfuric acid is lower than half that of 1 hydrochloric acid and sulfuric acid acts mostly as a monoprotic acid at the pH range employed the pK a.
In an experiment studying the clearance via the blood of radiolabeled sulfuric acid aerosol in different species the authors have observed that sulfur from sulfuric acid was rapidly cleared. The specific gravity of Sulphuric acid is 184 at 298 K. Ad Choose From 300000 Chemicals From Certified Manufacturers.
It has a density of 184 at 15 which does not fume. Great Deals Fast Shipping. Also it is diprotic and ionises in two stages in the aqueous solution.
Chemical Properties of Sulfuric Acid. Pure sulphuric acid is a colourless viscous liquid Density. It is completely soluble.
Sulfuric acid is a strong dibasic acid. Ad Bulk Chemical Resin Supplier. Being an acid sulfuric acid easily reacts with most bases and give the corresponding sulphate.

Sulfuric Acid Structure Formula Uses Facts Britannica

E141 Copper Complexes Of Chloropyll And Chlorophyllins Canned Fruits Chlorophyll Types Of Cheese
Physical Chemical Properties Of Sulphuric Acid Video Dailymotion
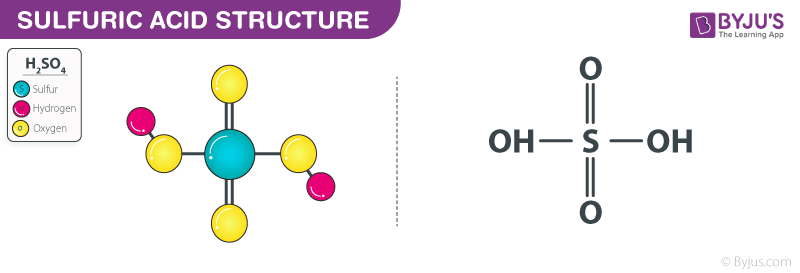
Sulfuric Acid H2so4 Structure Molecular Mass Properties Uses
0 Response to "Characteristics of Sulfuric Acid"
Post a Comment